|
|
Dr. Jiye Fang's Nano Research Group |
Single-atom catalysts (SACs) have become a frontier in catalysis as an attractive technique with exceptional performance, offering a promising platform for improving many key electrocatalytic and catalytic reactions, such as small organic molecule oxidation and ORR in fuel cells, OER and HER in water electrolysis, eCO2RR, water-gas shift (WGS) reactions (e.g., CO + H2O → CO2 + H2), and hydrogenation reactions. The supported SACs contain isolated individual atoms dispersed on, and/or coordinated with, surface atoms of appropriate supports, which not only maximize the atomic efficiency of metals but also provide an alternative strategy to tune the activity and selectivity of electrocatalytic and catalytic reactions.
In recent years, single-atom alloy (SAA) catalysts have also been shown to be powerful for a variety of catalytic reactions, such as selective hydrogenation reactions, dehydrogenation reactions, oxidation reactions, hydrogenolysis, and coupling reactions. The creation of SAA catalysts is based on the deposition of isolated reactive metal adatoms into host metal surfaces (of a relatively inert metal). The catalytic performance of SAA catalysts strongly depends on metal-support interactions and their composition and structure.
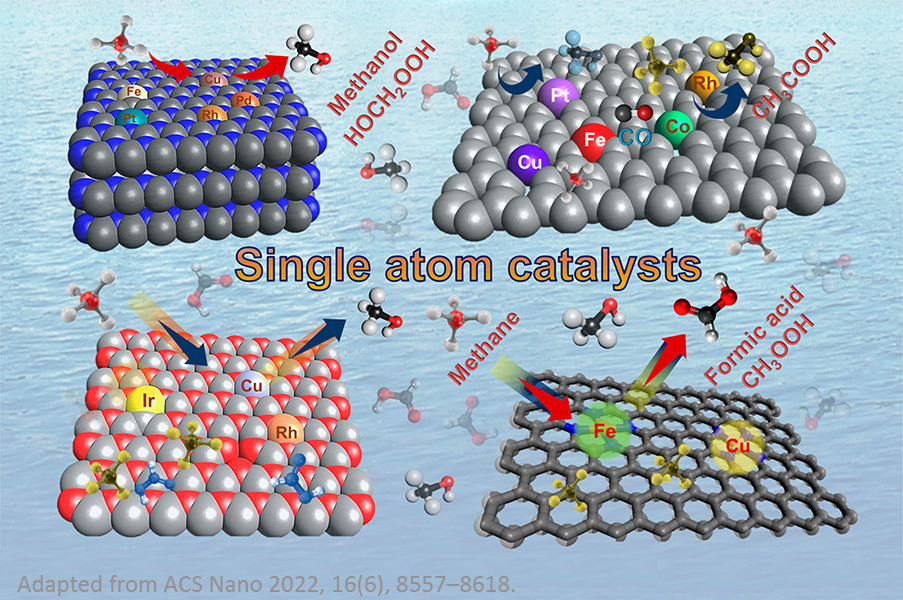
Further reading materials:
J. Mater. Chem. A, 13 (44) 38290–38300 (2025). 10.1039/D5TA05763A
Small Struct., 2 2000051 (2021). 10.1002/sstr.202000051
Binghamton University
· Chemistry
Department · 25 Murray Hill Road · Vestal, NY 13850
Materials on this website may only be browsed and saved for personal use.
Please do not reproduce and distribute commercially without permission from
the publisher or website owner.
 Meeting Schedule
Meeting Schedule Weekly Picks
Weekly Picks Research Training
Research Training Shared Resources
Shared Resources Chemical Inventory
Chemical Inventory Presentation Archive
Presentation Archive BU Brain
BU Brain Chem Stockroom
Chem Stockroom Internal Site
Internal Site MyBinghamton
MyBinghamton Group Posters
Group Posters Team Updates
Team Updates Joining our Lab
Joining our Lab Campus Map
Campus Map Campus Parking Info
Campus Parking Info|
|
||